In trying to understand electro-chemistry it is necessary to use so called electro-chemical “standard potential tables”. This requires a temporary diversion to explain what they are and illustrate their use – for rather trivial reasons they can be rather confusing. Fortunately by using the earlier analogy of water flowing between tanks we can introduce the concepts needed, clarify the use of the standard potential tables and introduce the electro-chemical series for voltaic cells mentioned earlier. Like all analogies, it has its limitations and some terms are used rather loosely.
A1. Background to understanding standard potential tables -flowing water again
A1.1 Spontaneous reactions
The flow of water from a hole at the bottom of a tank containing water kept at a constant level AB is shown schematically in figure 6. The flow is “spontaneous”: we do not need to do anything, gravity does it. The flow of water is driven by the gravitational potential, equal to the height H as measured from the reference position shown. The larger the height H, the greater is the flow of water out of the hole at the bottom.
Let’s call this downwards flow of water a “reaction”: it represents a real reaction which may take place in an electro-chemical half-cell, in this case the oxidation reaction for etching of a plate.
A1.2 Standard potential
The height “H”, the gravitational potential difference between the surface of the water and the reference level is a characteristic of the reaction: each reaction will have a different value of H. We will call this the standard potential for the reaction and place a + sign in brackets in front of it. The (+) simply means that the reaction is spontaneous.
For the electro-chemical equivalent of the standard potential, the reference “ level” is now a standard reference half-cell and the standard potential is numerically equal to the electric potential difference between the electrode in the half cell with the reaction of interest and the electrode in a standard half-cell. Such standard cells are only used for this purpose. In practice the voltaic or electrolytic cells will consist of two half cells with different reactions. Significantly and usefully, the standard potential for the overall cell may be obtained from the individual standard potentials. These details need not concern us but are mentioned for completeness.
A1.3 Electro-chemical series
Imagine two tanks both containing water at levels H1 and H2 respectively and connected together by a pipe. Water can flow spontaneously from the higher tank, larger standard potential H1 (say), to the tank below. If the water represents the flow of electrons from one plate in a half-cell through the external circuit to a plate in a second half-cell, we can see that the higher plate must be the anode and the lower one the cathode. We have passive etching of an anode or equivalently “sacrificial corrosion".
By making a table for the heights (standard potentials) H of the various half cells, with the cell with the largest standard potential at the top and the one with the smallest standard potential at the bottom – the electro-chemical series for metals - we have a means of deciding, for any pair of cells or plates, which plate is the anode (the plate etched): the one higher up in the table.

A1.4 Non-spontaneous reactions
The opposite or “reverse” reaction to water flowing downwards would be water flowing upwards from the reference level to a height H. Such a reaction would be non-spontaneous: water does not flow uphill without some assistance. This reverse reaction would be characterised by a standard potential (-) H. The (-) simply means the reaction is non-spontaneous: some energy, equivalent to the potential H, must be supplied in some way to push the water upwards a height H. The larger the value H, the more non-spontaneous is the reaction and the greater the energy which must be supplied.
A1.5 Characteristics of a non-spontaneous reaction
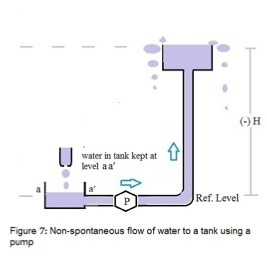
Since our interest is in active electro-etching, we will look at pumping water up to a tank at a height H above the reference tank, to examine the features of a non-spontaneous ”reaction”, shown schematically in figure 7. To raise water against gravity through a potential H so that the water flows over the top of the tank requires the pump P to have a pumping energy or “pumping potential” just greater than the gravitational potential H. The difference between the pump’s potential and the standard potential H is termed the “over-potential”. The larger the over-potential, the greater is the flow of water.
A1.6 Electro-chemical over-potential
For electro-chemical cells it is often found that an over- potential of only 0.2 - 0.4 volts above the standard potential is sufficient to produce significant electron current flow. The relation between the voltage over-potential and the electron current flow is given by the “Butler-Volmer” equation. Sometimes, however, the over-potential at which the electric current is first seen may be well above the value expected based on the standard potential –still less than a volt but large in electro-chemical terms. This is particularly true for reactions involving the generation of oxygen and hydrogen at the electrodes and partly explains why metallic ions may be deposited on the cathode in preference to the evolution of hydrogen gas.
A1.7 The possibility of more than one non-spontaneous reaction occurring.
Suppose two tanks at different heights, H1 and H2, with H2 larger than H1, are supplied with water by dividing the output from the pump. As the pumping potential is increased from zero, the tank at the lower height (smaller potential H1) will receive water once the pumping potential is greater than H1. As the pumping potential increases further so the over-potential for this tank gets larger, this increases the water flow to it. Only when the pumping potential is greater than H2 does water flow to the higher tank (larger potential H2).

Test print: texture obtained by pushing
fabric into the candle wax ground;
electro-etching, copper
The “reaction” which is the least non-spontaneous occurs first and although both reactions may be possible when the pumping potential is large enough, it is this reaction which is more “active” – has a larger flow of water. The same principle holds for a larger number of tanks/non spontaneous reactions.
It will be seen for electro-etching that by comparing the standard potentials for different reactions, some idea can be obtained as to which reactions will occur at the anode and cathode electrodes.
A1.8 Spontaneous and non-spontaneous reactions occurring together
Using the analogy of the last section, a spontaneous reaction can be thought of as one for which the height of the tank above the reference level is zero. Consequently, for all pumping potentials greater than zero, the pump will increase the flow of water for this spontaneous reaction while for the non-spontaneous reactions nothing will happen until the critical heights (standard potentials) are exceeded.
A2. From water pumping to electro–etching reactions
As discussed previously, the water pump is analogous to a battery or direct current power supply. The tanks represent half-cells in which various reactions take place. The flow of water between tanks represents the flow of electrons between electrodes through the outer circuit – the connecting wires. The water flow analogy does not consider any aspects of the electrolyte.


After Michelangelo
Stencil made by electro-etching through
a thin sheet of coated aluminium;
sodium carbonate electrolyte
Zn(s) → Zn(aq) ' '+ 2e− (+) 0.76 V Standard potential
The equivalent of the gravitational standard potential for a tank, the height H, is now the standard potential for a specific electro-chemical reaction in a half cell. We can treat the values of the electro-chemical standard potential in a similar way as we did for the gravitational analogy: (+) and (-) denote spontaneous and non-spontaneous reactions respectively.
A2.1 Some electro-chemical reactions – etching plates
Let’s look again at some reactions related to electro-etching. For example the electro-etching of zinc is given by the oxidation reaction:
The zinc plate which is the anode gives up electrons to the external circuit and zinc ions “dissolve” into the electrolyte. The (+) signifies that the reaction is spontaneous. This reaction is one of the half –cell reactions in the Daniell cell.
The reverse, reduction reaction is:
Zn(aq) ' '+ 2e− → Zn(s) (-) 0.76 V Standard potential
This reaction is non-spontaneous - the (-) sign. In this case, zinc from an electrolyte, say, of zinc sulphate would be deposited on the cathode plate. To obtain the reaction it would be necessary to make the cathode part of an electrochemical cell driven by a power supply.
Similar oxidation reactions apply for electro-etching aluminium and iron. They are spontaneous with standard potentials of (+) 1.66 V and (+) 0.44 V respectively. Copper does not follow this trend, its reduction reaction (depositing copper on a cathode), not its oxidation, etching reaction, is spontaneous with a standard potential of (+) 0.34 V. Hence passive etching of copper is only possible if the other electrode is silver, platinum or gold (see the electro-chemical series and standard potential tables).
A2.2 Standard potential tables
The example for zinc above shows that it is not necessary to write down both the oxidation and reduction reaction equations. If one reaction is known the reverse reaction is obtained from it by reversing the arrow in the equation and changing the sign in front of the standard potential.
Tables of standard potentials have been prepared for a wide range of possible electro-chemical reactions. By convention, they give the chemical equation and the (+) or (-) sign for the reduction reaction alone. A small selection is shown in the table below
Understanding the details of the electro-chemical equations shown is not critical, it is sufficient to appreciate the significance of the (+) or (-) sign and the relative values of the standard potentials for different reactions. However, it is worth emphasising what chemical equations, in general, do not tell us. An equation shows what in principle might happen but it does not give the required conditions, for example a high temperature may be necessary, and crucially the speed or rate at which the reaction occurs: it may be so slow that in practise it will never take place to any significant extent.
These values apply when the concentration of the solutions of ions or molecules is 1 mole per litre (1M) and the pressure of any gases present is 1 atmosphere.
Practical concentrations may often be different so consequently conclusions based on the standard potentials should only be taken as indicative. The actual potential in these circumstances can be found from the standard potentials by using the Nernst equation. Use is often made of so-called “Pourbaix diagrams” which provide a concise pictorial representation of the possible reactions and by-products for metal - water systems as a function of the cell potential, concentration and “pH” of the electrolyte, see section for an explanation of pH.
The spontaneous electro-etching reactions of zinc, aluminium and iron can in principle take place by passive etching in a voltaic cell using appropriate electrolytes and the correct combination of plates as obtained from the electro-chemical series.

Copper plate after electro-etching
in sodium chloride electrolyte
Alternatively, the plate to be etched can be made the anode plate in an electrolytic cell driven by a power supply - active electro-etching. Then, the action of the power supply is to increase the rate of these spontaneous reactions: it speeds up the etching.
The use of a power supply means that it is also possible for non-spontaneous reactions to take place if the applied voltage is larger than the required standard potential for the reaction(s). If both spontaneous and non-spontaneous reactions are possible at an electrode, the spontaneous reaction will be the preferred or dominant reaction with the non-spontaneous reaction possibly occurring as a secondary reaction, see sections A1.7 and A1.8.
A2.4 Gaseous electro-chemical reactions at electrodes
Of interest are the reactions involving the evolution of gases at the cathode or anode of a driven electrolytic cell.
At the anode:
Evolution of chlorine:
2Cl− (aq) → Cl2(g) (-)1.36 V
+2e−
Chlorine ions in the electrolyte give up electrons to the anode plate/external circuit and the chlorine atoms combine to form chlorine gas. The oxidation reaction is non-spontaneous.
Evolution of oxygen:
2 H2O → O2(g) + 4H'(aq) (-) 1.23 V (acidic)
+4e−
The reaction is non – spontaneous and comparing the value of its standard potential (-) 1.23 V with that for chlorine (-) 1.36 V it should be the preferred reaction since it is less non-spontaneous. However, the rate of reaction is relatively slow and at high concentrations of chlorine ions, chlorine not oxygen is liberated. Because H+ ions are produced at the electrode there is an excess of them and around the electrode the electrolyte solution can become acidic, see section 11.
At the cathode:
Evolution of hydrogen gas:
This can be achieved by the direct electrolysis of the water molecules or the combination of hydrogen ions which may be present in the aqueous solution; the equations below are for standard conditions:
The first reaction is the breaking up of water molecules to form hydrogen gas and hydroxide ions and it is non-spontaneous. The second reaction arises from the capture of electrons by positive hydrogen ions in the electrolyte and is the reaction used in the reference hydrogen cell. In each case the electrons are obtained from the cathode plate.
In an aqueous electrolytic solution, if it is acidic there are many more H+ ions than (OH) – ions and the second equation applies. If the electrolyte is alkaline the opposite is true. In general the potential for both cases can be represented by a single equation involving the pH, as shown, and is only zero under standard reference conditions. As the hydrogen is liberated at the cathode an excess of (OH) − ions are produced so that locally the solution may become alkaline, see section 11.
The relative simplicity of both the oxygen and hydrogen half-cell equations conceals the complex series of interactions which occur at the electrode plates to produce the gases and the sensitivity of the reactions to the conditions of the electrode surface, for example, smoothness, geometry, cleanliness and presence of impurities. This may partly account for the differing effects which may occur in different cells operating under the same conditions but with dissimilar electrodes.
A2.5 Why sodium and aluminium cannot be deposited onto a cathode
The reason that sodium and aluminium cannot be deposited onto the cathode of a driven cell from their ions in solution is because their standard potentials, (-) 2.71 V and (-) 1.66 V respectively, show that they are much more non-spontaneous than the generation of hydrogen, (-) 0.83 V maximum, which consequently is the preferred reaction.
A2.6 Why copper, zinc and iron can be deposited onto a cathode
The deposition of copper, zinc and iron are standard processes in the electroplating industry and with the appropriate electrolyte efficiencies of deposition are 95 -100 %. The standard potentials for deposition of copper, zinc and iron – a reduction reaction at the cathode - are (+) 0.34 V, (-) 0.76 V and (-) 0.44V respectively. Sulphate solutions of these metals would be weakly acidic, say a pH of 4. The relevant potential for the generation of hydrogen is then (-) 0. 24 V.
For copper the relative potentials show that deposition is preferred over hydrogen evolution but this does not appear to be the case for either zinc or iron. However for hydrogen evolving on a zinc cathode there is an additional over-potential of at least 0.6 V giving (-) 0.84 V and for hydrogen produced on an iron plate the over-potential is around 0.5 V giving (-) 0.74 V. Consequently, when the over-potential is considered, hydrogen is more non-spontaneous. Additional effects related to the relative concentrations of the metal and hydrogen ions may favour the deposition of the metal ions while the surface finish on the cathode may encourage hydrogen production: hydrogen evolution as a secondary cathode reaction is possible.
A2.7 Variation of current with voltage -passivity
The explanation of the electrolytic cell would suggest that as the applied voltage is increased so the current flowing through the cell and the rest of the circuit should increase. Sometimes it is possible to observe that the current remains constant or even decreases as the voltage is increased –a so-called passive region. The reasons for such behaviour are beyond the scope of this article but may be related to the inability of ions to reach the electrodes fast enough, the blocking of the electrode surface to ions due to the gases produced and the complexity of the reactions taking place.
Generally it should possible to find a current/voltage region with characteristics that allow etching at suitable currents. We mention the present effect for those rare occasions when you may find strange things happening during etching: it probably is not your fault!