Following an introductory overview, some physical and chemical principles relevant to electro-etching are introduced. No prior knowledge is assumed and no mathematics is involved.
Note: The “information” boxes provide additional details or comments of general interest and may be omitted on a first reading.
2. An overview of electro-etching
Essentially, the etching of the copper plate mentioned above is a result of the following electro-chemical process:
• Where the needled lines have broken through the ground, the surface of the copper plate is in contact with the liquid electrolyte;
• At these points of contact – the metal/liquid interface -and only at these points, the electrical energy obtained from the battery
enables some of the copper atoms to give up their outer shell electrons to the copper plate;
• These copper atoms thereby become positively charged copper ions Cu++ which break away from the surface of the copper to
become part of the liquid electrolyte;
• The amount of copper atoms which “dissolve” is proportional to the electric current flowing and the length of time the circuit is switched on.

Basic kit for “Home” electro-etching
1 -power supply based on using rechargeable batteries
2 -inexpensive multi-meter to monitor electric current
3 -polymer containers holding electrolyte
4 -electrodes and connecting wire
5 –plates to be etched
6 –tape and clips for masking and attaching plates to anode electrode
7 – polymer container for holding equipment during etching
8 – Dusk mask, gloves and safety glasses

The ionisation and transfer of the copper atoms from the plate to the neighbouring liquid, described as “dissolution” by electro-chemists, corrosion by material scientists, is more easily recognised by us as etching.
For electro-etching copper, zinc and iron it is possible to use two plates of the same material with an appropriate electrolyte which has a salt of the same metal – copper, zinc or iron sulphate – in such a way that the electrolyte remains essentially unchanged with successive etchings. A similar approach for etching aluminium does not work.
The key feature of electro-etching has been introduced: the atoms at the surface of a metal plate may give up some of their electrons to the external electric circuit to become positive ions which “dissolve” into the adjacent electrolyte. It now remains to fill in some of the details.
3. The material world – atoms and electrons
3.1 What we are made of – atoms; protons and electrons
Everything in the world is made from a combination of only a hundred or so substances, so-called elements, the majority of which are metals. The smallest amount of an element that can exist is an atom (from the Greek atomos “uncuttable”).
An atom is formed from just three sub-atomic particles, the proton, neutron and electron. The simplest atom belongs to the element hydrogen: it has a single electron orbiting around a nucleus consisting of a single proton. The proton possesses a “positive” electric charge while the electron has an equal but opposite “negative” charge so overall there is no excess charge –the atom is electro-neutral.
Positive and negative charges may sound rather abstract and remote but they are close by. The Greeks discovered electric charges –static electricity – by rubbing materials together – the triboelectric effect.
If you rub a balloon against your hair, and then place it just above your head, individual hairs will be attracted to the balloon but will be repelled by each other ( there are many excellent photos on the internet).
By the repeated contact, electrons have been transferred so that the hair has acquired positive charges (+) and the balloon negative charges (-) . Unlike charges (+/-) attract while like charges (-/- or +/+) repel. Long before the electron and proton were known, the terms positive and negative charge were introduced by Benjamin Franklin and were only intended to convey the fact that the charges had “opposite” effects, there was no mathematical implication.
The atomic structure of other elements can be built up from the basic hydrogen atom template by adding protons and neutrons to the nucleus and matching the number of protons by an equal number of electrons which orbit the central nucleus.
Atoms are very small; their radii are around 0.1 nm (10-10 metre) with the diameter of the central nucleus only about 10-14 metre. The weight of a hydrogen atom is 1.673 x 10-24g. The electric charge on an electron is 1.602 x 10-19 coulombs.
Large and small numbers: 109 means nine tens multiplied together – a million is 10 6
10-9 means 1 divided by nine tens – nanometre(nm)

3.2 The motion of electrons –orbits and shells
One way of visualising an atom uses a “solar system model”: the nucleus is the Sun and the electrons are the planets moving around the Sun in various orbits but with the difference that in a given orbit there can be more than one planet (electron). The permissible orbits and the number of electrons which can “occupy” a particular orbit or “shell” are governed by the rules of quantum physics, see figure 1.
3.3 Outer sub-shells determine chemical properties
The chemical properties of an atom and the way it may interact and combine with other atoms to form molecules are largely determined by the number of electrons occupying the outer shells/sub-shells where the influence of the nucleus is less. Some elements, for example the gas argon, have atoms which have just the right number of electrons to completely fill one or several shells/sub-shells to give a stable, low energy structure, making them inert and unable to react with other atoms.
Copper plate after electro-etching
in copper sulphate;
ground (candle wax) still on plate;
dark areas: natural “electro-tint”
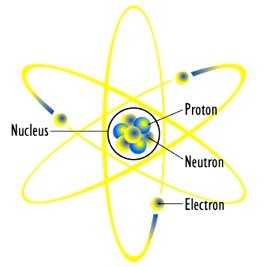
Figure 1: Rutherford “solar system model” of the atom, courtesy of Wikipedia article “Atom”
4. Metals
4.1 A basic property of metals
Characteristically, for metals the outer sub-shells will be occupied by only a few electrons, much less than the shell is able to accommodate. By contrast for many non-metals the outer sub-shells will be almost full of electrons.
4.2 A simple picture for the outer electrons within a metal – electric current
In a piece of metal there will be many millions of “outer” electrons which are essentially free to move within the open lattice, formed by the comparatively massive, immobile nuclei, and held together by the residue attractive electric forces between the “roaming” outer electrons and the nuclei. This may be pictured as a fixed array of buoys – the nuclei – bobbing on a “sea of electrons”. The passage of an electric current through a metal is really the flow of these electrons.
4.3 Sharing electrons and the formation of ions
Metal atoms would be more stable and “happier” if they had a structure similar to argon, that is, fully occupied shells/subshells. They can achieve this under the “right conditions” by donating their electrons in the outer sub-shells to a non-metal which correspondingly would like to gain the same number of electrons to complete its outer sub-shell so that it also has an argon-like structure.
Before the discovery of the electron it was incorrectly thought by Faraday and Franklin that the flow of electric current was due to the flow of positive charge from the positive terminal of a battery, through the circuit to the negative terminal. This convention is still used today with the recognition that such a “conventional” current is equal and opposite to the actual electron current.
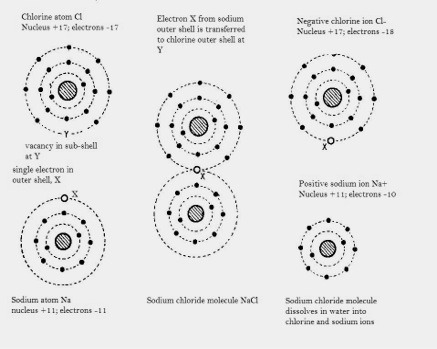
Figure 2: Formation of a sodium chloride molecule from sodium and chlorine atoms
and the formation of chlorine and sodium ions by dissolving sodium chloride in water.
The atom of metallic sodium (Na) has its inner shells full leaving just one electron in its third shell - capable of holding eighteen electrons. The atom of the non-metal chlorine (Cl) again has its inner shells full, leaving seven electrons occupying the third shell, one electron short of filling two of its sub-shells.
By transferring an electron from the sodium atom to the chlorine atom both achieve complete sub-shells. The atoms Na and Cl become positively and negatively charged ions Na+ and Cl -.The electric force of attraction binds these ions together and leads to a lattice type structure incorporating huge numbers of atoms to give the familiar crystals of common salt.
A metal atom, in transferring electrons to another atom ceases to be a neutral atom - it has excess positive electric charge because there are more protons in the nucleus than there are orbiting electrons – it has become a positive ion. Similarly, the non-metal atom which has received the electrons now has excess negative charge; it has become a negative ion. The positive and negative ions thus formed become bound to one another through the attractive electric forces –ionic bonding.
The neutral chlorine atom Cl, because it has a space left in its outer sub-shell, has a great desire to combine with other atoms –it is “highly reactive”; a so-called “ radical”. By contrast the chlorine ion Cl− which has gained the required electron generally forms stable ionic salts. It is remarkable that sodium chloride, common salt, essential to life, is composed from two dangerous elements.
4.4 Metals for etching and their ions
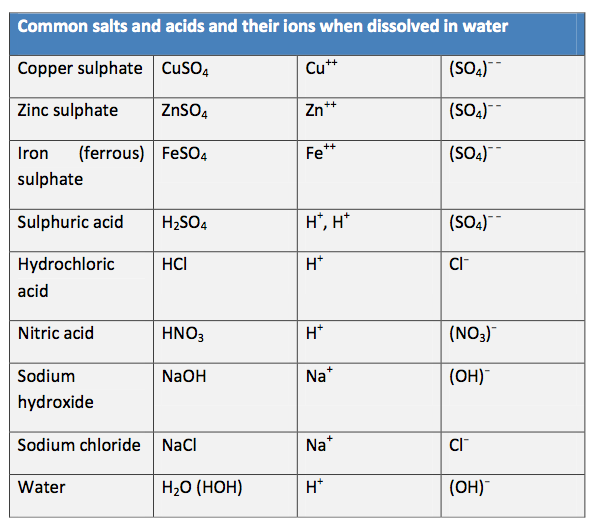
The electron distribution for atoms of aluminium, zinc, iron and copper –the metals of interest for etching -are such that the following ions would be expected if electrons in the outer shells can be lost to form a more stable ion:
5. Electrolytes
5.1 Combining atoms - salts
Elements seldom exist as single atoms, rather they combine to form compounds some of which consist of thousands of atoms. Of particular relevance for us are the compounds belonging to the class called salts, for example sodium chloride NaCl, common salt, copper sulphate CuSO4 and iron sulphate FeSO4 - each contains a metal atom and a sulphate molecule held together by ionic bonding. These salts are dissolved in water to form the liquid electrolytes for electro-etching.

Test print: line drawing, copper plate, candle wax ground,
electro-etch in copper sulphate; after Rembrandt
A picture of the everyday occurrence of table salt dissolving in water, viewed at the atomic level, has only been revealed as recently as 2011 with the aid of simulations using super computers, see YouTube “How does salt dissolve in water ?”.
It is convenient to represent the elements, compounds and the reactions between them in a short-hand notation using symbols, formulae and equations. An atom of an element is given a symbol, so for example, H stands for a hydrogen atom, O for an oxygen atom, while H2O represents one molecule of water comprising two hydrogen atoms and one atom of oxygen – the subscript gives the number of atoms; two molecules of water would be represented by 2 H2O.
Positive ions may be represented as Cu++ or Cu 2+ where the superscript ++ or 2+ stands for two positive charges. Similarly for negative ions: Cl− and (SO4) − − which is a sulphate ion (one sulphur atom and four oxygen atoms) with two excess negative charges. Electrons always have a single negative charge and may be represented by e or e−.
An equation can be used to represent a reaction and must obey certain rules to ensure that the total number of atoms and excess charges balance but that need not concern us. Some examples are:
2Cl− (aq) → Cl2(g) +2e−
Two negative ions of chlorine in liquid aqueous solution (2− charges) react (reaction details are not given by the equation) to give one molecule of chlorine gas and two electrons; often the states –liquid, gas, solid - are omitted.
Zn(s) + Cu++(aq) →Zn− − (aq) + Cu(s)
This equation describes the reaction when a piece of zinc is placed in an aqueous solution of copper sulphate CuSO4.The copper sulphate splits into a positive ion Cu ++ and a negative sulphate ion(SO4) − − but since the sulphate ion and the water play no part in the reaction they are not shown. Similarly the transfer of two electrons from the zinc to the copper is not shown. This overall reaction can be understood more easily if it is split into two “half reactions”:
Zn → Zn ++ +2e − oxidation reaction
a zinc atom is ionised releasing two electrons;
Cu++ +2e − → Cu reduction reaction
copper ions in the copper sulphate solution capture the two electrons released by the zinc atom to form a copper atom.
Such equations describing chemical reactions show how many atoms or molecules of each substance takes part in the reaction, they also show how many moles of each substance take part.
Experimentally we do not deal with a single atom or molecule but with moles of atoms or molecules. A mole is just a quantity –a number rather like a dozen – known as Avogadro’s number 6 x 10 23, that is 6 multiplied by 23 tens, a rather large number!
The weight of a mole of atoms or molecules in grams is known as the gram atomic or molecular weight. A molar solution is one in which the gram atomic or molecular weight of a substance is dissolved in sufficient water to make one litre of solution, written as 1M solution.
5.2 Dissolving salts in water –electrolytes for electro-etching; electro-neutrality
Salts dissolve in water because the water molecules are able to break up the ionic lattice into positive and negative ions. The number of negative and positive charges is equal so the solution as a whole is “electro-neutral”- zero net charge.
A general principle is that nature, left to itself, discourages processes which create excess electric charge, it prefers electro-neutrality.
5.3 Electric current in a liquid electrolyte; salt bridge
In an electrolyte there are no free electrons to provide the electric current. Instead the current is due to the movement of the positive and negative ions. For current to flow from one container of electrolyte to another, a salt bridge may be used.
A salt bridge is typically an inverted glass U-tube with each end dipping into one of the containers. It is filled with an appropriate electrolyte with porous plugs at each end. These plugs allow the ions from the electrolyte in the salt bridge to pass through into the containers but prevent the ions in the containers from flowing into the bridge; see the later image of the Daniell cell.